Multiparametric Approach to Quality by Design (QbD)
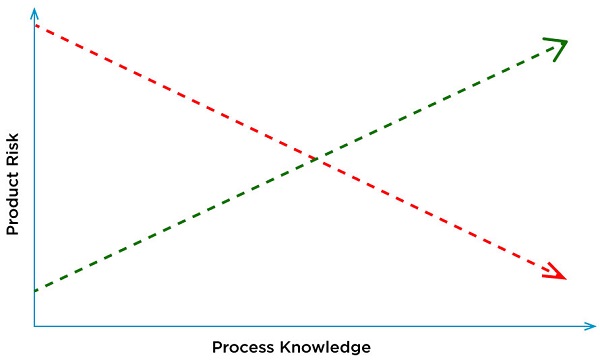 Using living cells provides a more physiological response. A challenge in designing and executing cell-based assays is identifying and controlling variability. As living entities, cells sense and respond to their environment. Simple manipulations can cause them to change their properties. A poorly controlled assay can cause an manufacturing process to appear out of control or result in invalid or re-test scenarios. QbD decreases the product risk and increases process knowledge using a scientific framework.
Using living cells provides a more physiological response. A challenge in designing and executing cell-based assays is identifying and controlling variability. As living entities, cells sense and respond to their environment. Simple manipulations can cause them to change their properties. A poorly controlled assay can cause an manufacturing process to appear out of control or result in invalid or re-test scenarios. QbD decreases the product risk and increases process knowledge using a scientific framework.
Flow cytometry can provide multiparametric data at single-cell resolution enabling simultaneous monitoring of Critical Method Parameters (CMAs) and interrogating complex interrelated responses. Assessing cellular responses on multiple levels can lead to more efficient identification of CQAs.
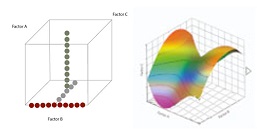
One factor at a time (OFAT, panel A) versus the multifactorial Design Of Experiment (DoE, panel B) better identifies efficient design space for QdB analysis.
Flow Cytometry Assays
At Beckman Coulter Life Sciences, our flow cytometry division has been developing flow cytometry assays for highly regulated clinical diagnostic testing. Our knowledge of robust assay design and a focus on the workflow positions us as a strong partner for developing flow cytometry-based solutions for complex cell-based assays. Mitigating sources of variability begins during assay design, continues during QbD analytical method development, and through process monitoring.
We are committed to providing tools to improve the quality of data used to make critical decisions in process development through to batch release testing.
- Reduce labor intensive administrative tasks
- Reduce pipetting errors and reagent inventory management
- Facilitate compliance with quality system and regulatory requirements
- Reduce subjectivity in data analysis and manage data securely
Flow Cytometry Assays by Development Stage and Regulatory Framework
| Drug Discovery | Non-clinical Testing | Clinical Testing |
| Non-Regulated Target identification Mechanism of action Biomarker identification |
GLP GMP |
IVD or CLIA GLP GMP |
Products