Host Cell Residual DNA Testing in Reduced Volume qPCR Reactions Using Acoustic Liquid Handling
Jared Bailey and John LesnickAbstract
Testing for residual host cell DNA throughout the biopharmaceutical manufacturing process is an important step for product safety and lot validation. The Applied Biosystems resDNASEQ Residual DNA Quantitation Kit allows for accurate qPCR testing of multiple host cell backgrounds but requires challenging serial dilutions and a large reaction volume to function correctly. By integrating the resDNASEQ workflow with Echo Liquid Handlers, the number of serial dilutions were dramatically reduced, the reaction volumes were lowered, and with a dispense resolution of 2.5 nL, an increase in DNA sensitivity was observed. The precise and accurate acoustic transfer ability afforded by the Echo Liquid Handler platform greatly enhances the residual host cell DNA testing workflow.
Introduction
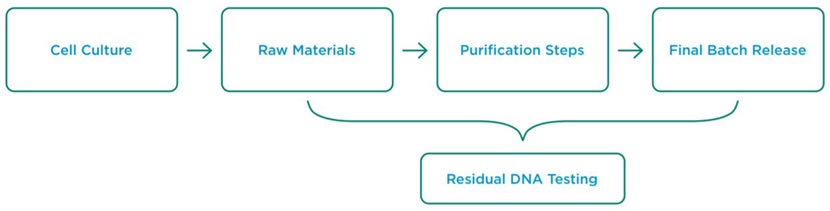
The removal of host cell impurities is a critical step in the production of biopharmaceutical products. One impurity targeted for clearance during the process is residual DNA arising from host cells. Testing for this impurity occurs at several steps throughout production (Figure 1). In addition to potential safety issues associated with extraneous host cell DNA, the regulatory guidance for products produced in cell culture specifies that DNA content in the final product should be as low as possible, as determined by a highly sensitive method. Traditional methods of quantitating residual host cell DNA have been limited by laborious sample preparation protocols, and lack of sensitivity. The Applied Biosystems resDNASEQ Residual DNA Quantitation Kit is a quantitative PCR (qPCR)-based system for the detection of this residual DNA from a variety of host cell lines that overcomes these hurdles. Specific and sensitive results are possible with the included TaqMan probes being capable of detecting as little as 0.03 pg of host cell DNA. Kits have been made with probes specific for a wide range of cell lines including those from E. coli, human, and Chinese hamster ovary (CHO) origin.
Figure 1: Typical residual DNA testing points during biopharmaceutical production.
Figure 2: The Echo 655T, 555, and 525 Liquid Handlers
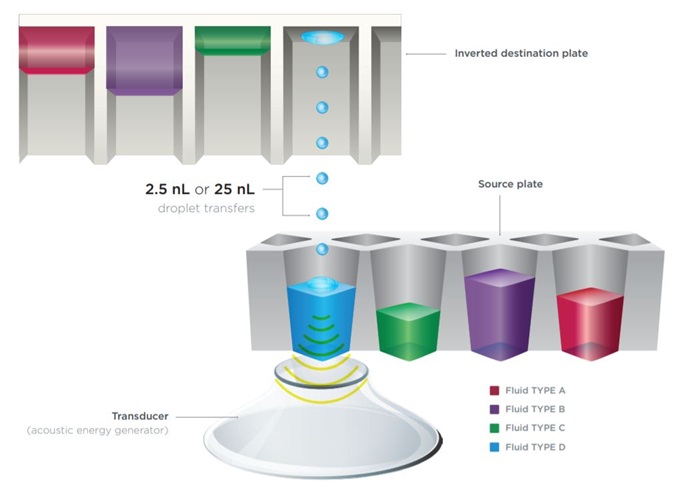
While the chemistry provided by the resDNASEQ Kit is excellent, workflow improvements can still be made through the use of an accurate liquid handler. To that end, testing of the resDNASEQ system was performed on three different liquid handlers manufactured by Beckman Coulter Life Sciences; the Echo 655T, 555, and 525 Liquid Handlers all use acoustic energy to precisely transfer small volumes of liquid between a source and destinationplate (Figure 2).
The latest in Echo Liquid Handlers, the Echo 655T Liquid Handler is part of the Echo 650 Series systems, with upgraded fluidics, streamlined architecture, and the option of transfer from acoustic tubes. Both the Echo 655T and Echo 555 Liquid Handlers generate 2.5 nL droplets at a rapid rate allowing for a high level of granularity during reaction setup. The Echo 525 Liquid Handler, which was also applied to the workflow, generates 25 nL drops.
Due to the 2.5 or 25 nL dispense volume resolution afforded by Echo Liquid Handlers, numerous cellular, biochemical and molecular biological techniques have been miniaturized by decreasing reagent requirements. The manufacturer recommended total qPCR reaction volume for resDNASEQ Kits is 30 μL. This volume can be largely attributed to guarding against the inaccuracy of human pipetting of sub-microliter reagent amounts. Beyond the overall lowering of reagent cost, the Echo Liquid Handlers allow for a reduction in cross-contamination due to the lack of any physical interaction with the liquid. The nanoliter scale of the Echo 655T, 555, and 525 Liquid Handlers allows for a qPCR standard curve to be generated by transferring DNA directly to all plate wells, instead of sequentially diluting across the plate. Echo Liquid Handler enabled direct dilution improves reliability and data quality by eliminating numerous, error prone, serial dilutions. qPCR reactions can be miniaturized effectively with the resDNASEQ Kits in combination with an Echo Liquid Handler.
Figure 3: Both the Echo 655T and 555 Liquid Handlers transfer discrete 2.5 nL droplets between a source and an inverted destination plate, while the Echo 525 Liquid Handler produces 25 nL droplets. Multiple fluid types are possible on all the instruments allowing for the entire reaction to be set up on a single source plate.
Methods
The resDNASEQ Quantitative Kits for testing residual host-cell DNA from E. coli, human, and CHO cells were ordered directly from Thermo Fisher Scientific. Each of the kits provide a positive control DNA standard of genomic DNA for the designated host target. These standards were diluted into four stock concentrations to allow for overlapping acoustic DNA additions between 3 x 104 and 3 x 105 pg as shown in Table 1.
The Master Mix was created by manually adding 92 µL of water with 138 µL of the appropriate 10X DNA assay mix, and 690 µL of the 2X Environmental Master Mix into a 1.5 mL microcentrifuge tube.
The tube was gently vortexed before being pipetted into wells of an Echo Qualified 384-Well Polypropylene Microplate. From this plate, the Master Mix and other reagents listed in Table 1 were acoustically arrayed in triplicate into a Bio-Rad Hard-Shell 480 384-well PCR plate to a final reaction volume of 5 μL. The order of acoustic addition was Master Mix, diH20 Backfill, and lastly DNA. Three PCR plates were generated from the same stock dilutions using either the Echo 655T Liquid Handler, the Echo 555 Liquid Handler, or the Echo 525 Liquid Handler to dispense all the reagents.
|
Echo 655T, 555, and 525 Liquid Handlers Dispensing Volumes Six-fold Reduced resDNASEQ Kit qPCR Reactions |
||||||||
| Intermediate dilutions of positive control DNA | Volume DNA Added (nL) | diH20 Backfill (nL) | MasterMix (10X DNA assay mix included) (nL) | Final DNA amount per reaction (pg) | ||||
| Echo 555 and 655T Liquid Handlers | Echo 525 Liquid Handler | Echo 555 and 655T Liquid Handlers | Echo 525 Liquid Handler | Echo 555 and 655T Liquid Handlers | Echo 525 Liquid Handler | Echo 555 and 655T Liquid Handlers | Echo 525 Liquid Handler | |
| High Concentration DNA (1.8 ng/ µL ) | 1667.5 | 1650 | 0 | 0 | 3332.5 | 3350 | 3001.5 | 2970 |
| 167.5 | 165 | 1500 | 1485 | 3332.5 | 3350 | 301.5 | 297 | |
| 17.5 | 25 | 1650 | 1625 | 3332.5 | 3350 | 31.5 | 45 | |
| 2.5 | 1665 | 3332.5 | 4.5 | |||||
| Mid-High Concentration DNA (.018ng/ µL) | 1667.5 | 1650 | 0 | 0 | 3332.5 | 3350 | 30.015 | 29.7 |
| 167.5 | 165 | 1500 | 1485 | 3332.5 | 3350 | 3.015 | 2.97 | |
| 17.5 | 25 | 1650 | 1625 | 3332.5 | 3350 | 0.315 | 0.45 | |
| 2.5 | 1665 | 3332.5 | 0.045 | |||||
| Mid-Low Concentration DNA (.00018 ng/ µL) | 1667.5 | 1650 | 0 | 0 | 3332.5 | 3350 | 0.30015 | 0.297 |
| 167.5 | 165 | 1500 | 1485 | 3332.5 | 3350 | 0.03015 | 0.0297 | |
| 17.5 | 25 | 1650 | 1625 | 3332.5 | 3350 | 0.00315 | 0.0045 | |
| 2.5 | 1665 | 3332.5 | 0.00045 | |||||
| Low Concentration DNA (.0000018 ng/ µL) | 1667.5 | 1650 | 0 | 0 | 3332.5 | 3350 | 0.0030015 | 0.00297 |
| 167.5 | 165 | 1500 | 1485 | 3332.5 | 3350 | 0.0003015 | 0.000297 | |
| 17.5 | 25 | 1650 | 1625 | 3332.5 | 3350 | 0.0000315 | 0.000045 | |
Table 1: DNA direct dilution scheme for the resDNASEQ Quantitative E. coli, human, and CHO kits using four different intermediate control concentrations to allow for overlapping concentrations. The initial “High Concentration” dilution was made from the manufacturer provided 30 ng/μL standard with the lower three dilutions being generated from a manually-pipetted serial dilution. Each final DNA amount was set up in a triplicate reaction. For the Echo 655T and 555 Liquid Handlers, the 384PP_AQ_GP3 calibration was used to acoustically dispense the DNA and water while the 384_AQ_CP calibration was used for the Master Mix addition. For the Echo 525 Liquid Handler, 384_PP_Plus AQ_GPSA calibration was used to transfer the Master Mix, and 384_PP_AQ _BP calibration used for the water and DNA additions. The highlighted rows are the manufacturer given limit of detection.
A fourth test PCR plate was generated to test the effect of miniaturized reaction volumes on a complex growth medium input. To accomplish this, an aliquot was taken from sub-confluent CHO cells during log growth. These cells were grown in Ham’s Nutrient Mixture F12 supplemented with 10% Fetal Bovine Serum. Upon sample removal, the DNA was quantified using a Qubit 2.0 Fluorometer with a Qubit dsDNA HS Assay Kit. The undiluted aliquot was approximated to have 92 pg of DNA. A 1 to 100 dilution was made, and both the undiluted and diluted samples were transferred using the direct dilution capability of the Echo 655T Liquid Handler into 5 μL reactions (Table 2).
|
Echo 655T Liquid Handler Dispensing Volumes Six-fold Reduced CHO Cell resDNASEQ Kit qPCR Reactions |
||||
| Intermediate dilutions of cell culture | Volume aDNA Added (nL) | diH20 Backfill (nL) | MasterMix (10X DNA assay mix included) (nL) | Final DNA amount per reaction(pg) |
| High Concentration CHO Cell DNA (~92 pg/µL) | 1000 | 667.5 | 3332.5 | 92 |
| 500 | 1167.5 | 3332.5 | 46 | |
| 100 | 1567.5 | 3332.5 | 9.2 | |
| 75 | 1592.5 | 3332.5 | 6.9 | |
| 25 | 1642.5 | 3332.5 | 2.3 | |
| 2.5 | 1665 | 3332.5 | 0.23 | |
| High Concentration DNA (~0.92 ng/µL) | 1667.5 | 0 | 3332.5 | 1.53 |
| 1250 | 417.5 | 3332.5 | 1.15 | |
| 1000 | 667.5 | 3332.5 | 0.92 | |
| 500 | 1167.5 | 3332.5 | 0.46 | |
| 250 | 1417.5 | 3332.5 | 0.23 | |
| 100 | 1567.5 | 3332.5 | 0.092 | |
| 25 | 1642.5 | 3332.5 | 0.023 | |
| 2.5 | 1665 | 3332.5 | 0.0023 | |
Table 2: DNA direct dilution scheme for the CHO cell testing by the resDNASEQ Quantitative CHO kit using two different dilutions. The 384PP_AQ_GP3 calibration was used to acoustically dispense the DNA and water while the 384_AQ_CP calibration was used for the Master Mix addition.
Upon acoustic transfer completion, each of the PCR plates was sealed with Bio-Rad Microseal ‘C’ PCR Plate Sealing Film. The plates were then briefly vortexed for 15 seconds at 2000 RPM on an Eppendorf MixMate. After vortexing, the plates were centrifuged for 2 minutes at 1000 x g and immediately loaded into a Roche Lightcycler 480 II. Thermocycling conditions were carried out according to the manufacturer recommended conditions as shown in Table 3.
After the thermocycling run was completed, the plates were analyzed using LightCycler 480 Software. Analysis was accomplished using the absolute quantitation second derivative algorithm. The derived CP values were subsequently imported into Graphpad Prism for further analysis and visualization.
| Thermocycling Conditions for the resDNASEQ Quantitative Kits | |||
| Step | Cycles | Temperature (°C) | Time (Min:Sec) |
| AmpliTaq Gold enzyme activation | 1 | 95 | 10:00 |
| PCR | 40 | 95 | 0:15 |
| 60 | 1:00 | ||
| Cool | 1 | 37 | Hold |
Table 3: Thermocycling conditions for the resDNASEQ Quantitative Kits on the Roche Lightcycler 480 II. The FAM filter was used for data acquisition at the end of each of the 60°C anneal/ extensions steps.
Results
I. E. coli resDNASEQ Quantitative DNA Kit
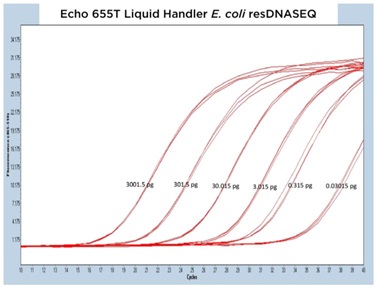
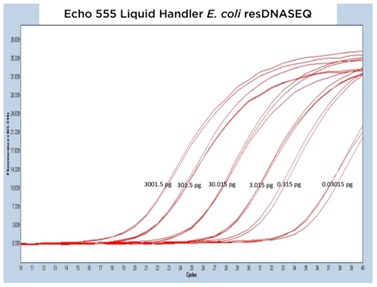
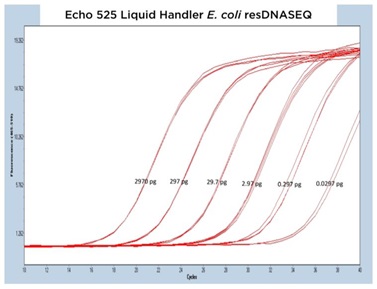
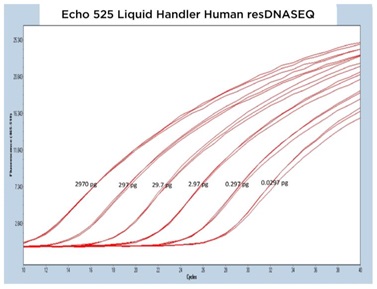
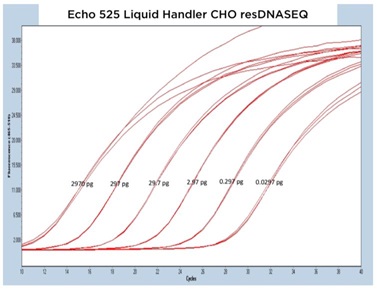
The results from each of the different resDNASEQ assays have been grouped by host target to better demonstrate the consistent performance of the Echo Liquid Handler. In Figure 4A, 4B, and 4C, qPCR traces are shown for the Echo 655T, 555, and 525 Liquid Handlers, respectively.
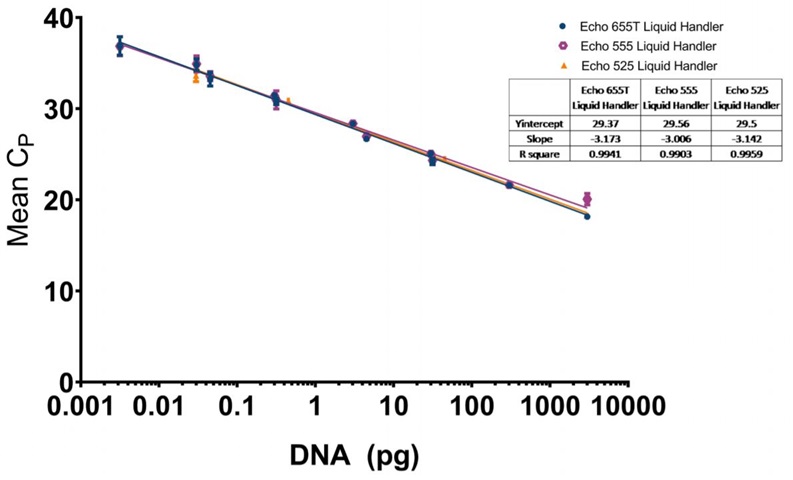
The data generated is extremely reproducible with Echo 655T Liquid Handler and Echo 555 Liquid Handler dispensed reagents having an average CP percent CV of 1.10% and 1.59% respectively to the 0.003 pg concentration. When the mean CP from each triplicate sample down to the 0.003 pg concentration are graphed for the Echo 655T and 555 Liquid Handlers on a semi-log graph, both trend lines are shown to be extremely reproducible (Figure 4D) The 0.003 pg DNA quantification represents an extra order of magnitude from the manufacturer lower limit of quantitation (LLOQ) of 0.03 pg. For the Echo 525 Liquid Handler, the CP percent CV was 0.76% to the manufacturer proscribed 0.03 pg level (Figure 4D) when the replicates were compared. The Echo 525 Liquid Handler was capable of quickly filling the PCR plates while maintaining the manufacturer specified kit limitation of quantitation of 0.03 pg.
4A. |
4B. |
4C. |
Figure 4 (A–C): A, B, and C: Representative qPCR traces of triplicate technical replicates for the E. coli resDNASEQ Quantitative Kit. The results are from reagents dispensed by either the Echo 655T, 555, or 525 Liquid Handlers. Results are shown across multiple orders of magnitude demonstrating reproducibility. Quantity of DNA per reaction is annotated in picograms. |
Echo Liquid Handler Enabled E. Coli resDNASEQ qPCR
4D.
Figure 4 (D):
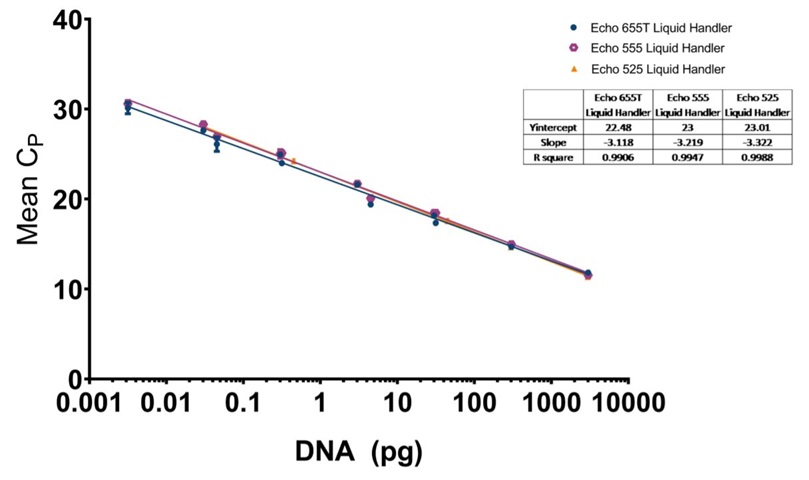
D: E. coli resDNASEQ qPCR standard curve for reagents dispensed by the Echo 655T (Blue Circle), 555 (Purple Octagon), or 525 (Orange Triangle) Liquid Handler with a logarithmic X-axis for DNA concentration, and mean CP values plotted on the Y-axis. The CP mean was calculated from triplicate technical replicate samples as mentioned in the methods. Each point has standard deviation error bars with an overall R2 value of 0.9941 for the Echo 655T Liquid Handler, 0.9903 for the Echo 555 Liquid Handler, and 0.9959 for the Echo 525 Liquid Handler. The manufacturer recommended lower limit of 0.03 pg in a 30 μL reaction was reached by all three instruments in a 5 μL reaction and was capable of being lowered to 0.003 pg by the Echo 655T and 555 Liquid Handlers.
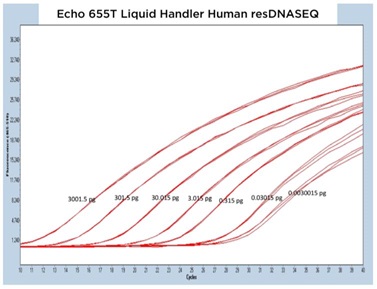
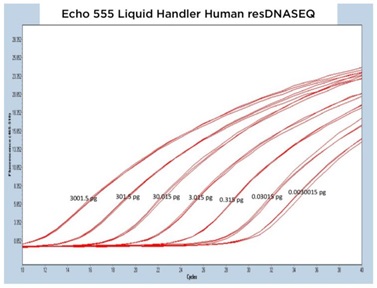
II. Human resDNASEQ Quantitative DNA Kit
The human resDNASEQ standard produced similar results to the E. coli kit albeit at a lower CP for the DNA amounts tested. Lightcycler 480 traces for the three Echo Liquid Handlers produced similarly shaped curves over three separate test plates (Figure 5A, 5B, and 5C). The human DNA dilution standards down to the 0.003 pg level transferred with the Echo 655T were even more precise than the E. coli with a mean percent CV of 0.76% for the CP of each technical replicate and the Echo 555 Liquid Handler also had a tight data set with a mean percent CV of 1.09 % (Figure 5D). As with the E. coli kit, the Echo 525 Liquid Handler transferred human resDNASEQ reagents reached the manufacturer specification of 0.03 pg, but with a low mean percent CV of 0.46% when looking at the triplicate CP values.
5A. |
5B. |
5C. |
Figure 5 (A–C): A, B, and C: Representative qPCR traces of triplicate technical replicates across multiple orders of magnitude of DNA concentration for the Human resDNASEQ Quantitative Kit. The results are from reagents dispensed by either the Echo 655T, 555, or 525 Liquid Handlers. Quantity of DNA per reaction is annotated in picograms. |
Echo Liquid Handler Enabled Human resDNASEQ qPCR
Figure 5 (D):
D: Human resDNASEQ qPCR standard curve for reagents dispensed by the Echo 655T (Blue Circle), 555 (Purple Octagon), or 525 (Orange Triangle) Liquid Handler with a logarithmic X-axis for DNA concentration, and mean CP values plotted on the Y-axis. The CP mean was calculated from triplicate technical replicate samples as mentioned in the methods. Each point has standard deviation error bars with an overall R2 value of 0.9941 for the Echo 655T Liquid Handler, 0.9903 for the Echo 555 Liquid Handler, and 0.9959 for the Echo 525 Liquid Handler. The manufacturer recommended lower limit of 0.03 pg in a 30 μL reaction was reached by all three instruments in a 5 μL reaction and was capable of being lowered to 0.003 pg by the Echo 655T and 555 Liquid Handlers.
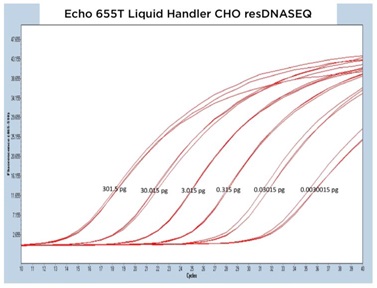
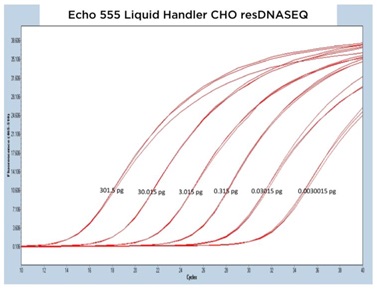
III. Echo Liquid Handler Enabled CHO resDNASEQ qPCR
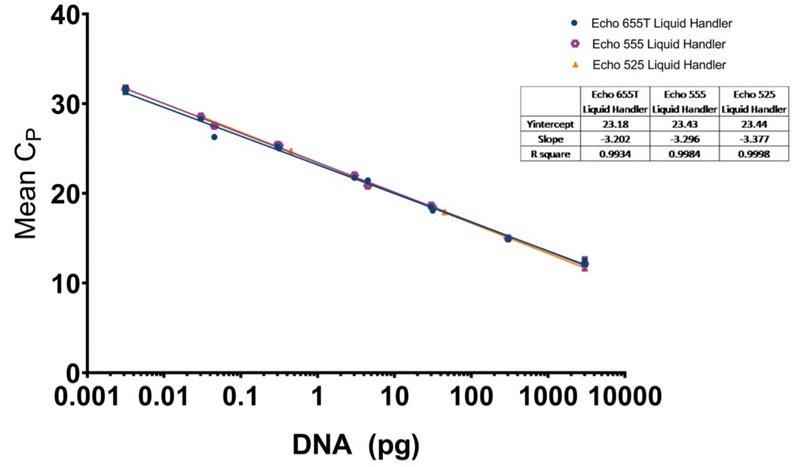
The CHO resDNASEQ Quantitative DNA Kit was the third host-cell residual DNA system tested on the Echo Liquid Handlers in this study. The representative qPCR traces shown in Figure 6A, 6B, and 6C are again very reproducible between DNA standard replicates. The Echo 655T Liquid Handler demonstrated a percent CV of 0.64% when the replicate CP values were averaged, and the Echo 555 Liquid Handler had a percent CV of 0.88%. These numbers were able to again be reached even with an increased order of magnitude from the manufacturer claimed LLOQ to 0.003 pg DNA. The Echo 525 Liquid Handler demonstrated a percent CV of 0.24% when the CP values were compared down to the 0.03 pg DNA amount.
6A. |
6B. |
6C. |
Figure 6 (A–C): A, B, and C: Representative qPCR traces of triplicate technical replicates across multiple orders of magnitude of DNA concentration for the CHO resDNASEQ Quantitative Kit. The results are from reagents dispensed by either the Echo 655T, 555, or 525 Liquid Handlers. Quantity of DNA per reaction is annotated in picograms. |
Echo Liquid Handler Enabled CHO resDNASEQ qPCR
6D.
Figure 6 (D):
D: CHO resDNASEQ qPCR standard curve for reagents dispensed by the Echo 655T (Blue Circle), 555 (Purple Octagon), or 525 (Orange Triangle) Liquid Handler with a logarithmic X-axis for DNA concentration, and mean CP values plotted on the Y-axis. The CP mean was calculated from triplicate technical replicate samples as mentioned in the methods. Each point has standard deviation error bars with an overall R2 value of 0.9934 for the Echo 655T Liquid Handler, 0.9984 for the Echo 555 Liquid Handler, and 0.9998 for the Echo 525 Liquid Handler. The manufacturer recommended lower limit of 0.03 pg in a 30 μL reaction was reached by all three instruments in a 5 μL reaction and was capable of being lowered to 0.003 pg by the Echo 655T and 555 Liquid Handlers.
IV. CHO resDNASEQ Quantitative DNA Kit with Cell Culture
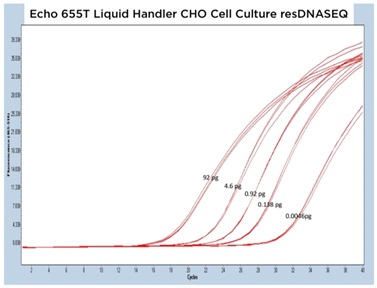
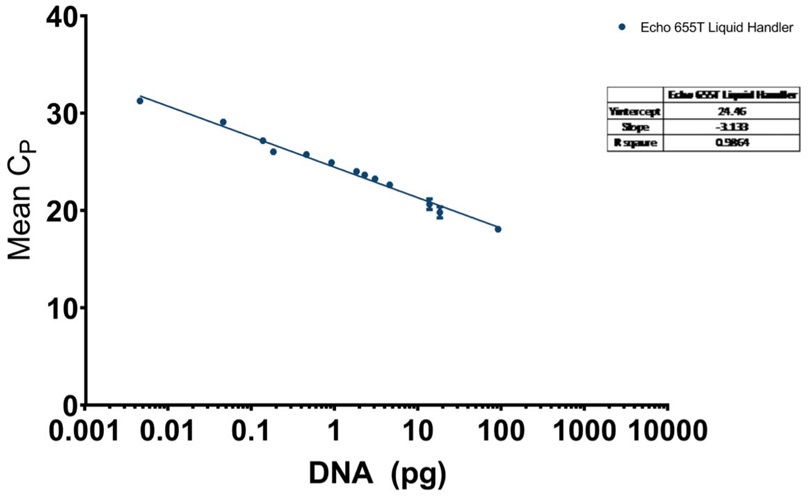
Beyond testing with the manufacturer provided genomic DNA, a cell culture sample had been tested. In Figure 7A, the qPCR traces show that there was consistent acoustic transfer of the complex media. The Echo 655T was used to make multiple dilutions, and these are well captured on Figure 7B. There, when the CP values are plotted against the DNA amount on a semi-logarithmic graph, a linear relationship can be seen with an R2 of 0.9884. The mean CP CV% was 0.74% between points.
7A.
Figure 7:
A: Representative qPCR traces for triplicate CHO cell culture samples dispensed into resDNASEQ reagents by the Echo 655T Liquid Handler. Results are shown across multiple orders of magnitude demonstrating reproducibility. Approximate quantity of DNA per reaction is annotated in picograms and were gathered from serial dilutions made from a Qubit 2.0 Fluorometer quantitated sample.
B: CHO resDNASEQ qPCR standard curve for reagents dispensed by the Echo 655T Liquid Handler with a logarithmic X-axis for DNA concentration, and mean CP values plotted on the Y-axis. Each point has standard deviation error bars with an overall R2 value of 0.9864.
Echo Liquid Handler Enabled Human resDNASEQ qPCR
7B.
Conclusion
Thermo Fisher Scientific’s resDNASEQ system is a valuable tool to quantitate host cell DNA contamination for numerous cell backgrounds. However, the kit is expensive partially due to the large, 30 µL total reaction volume that is required to accurately quantitate DNA down to the specified 0.03 pg when manually arraying DNA. When combined with the acoustic dispensing capabilities of Echo Liquid Handlers, this kit is capable of being reduced six-fold to a total reaction volume of 5 µL. This volume was attainable in all the standard curves generated, as well as with an assessment of a cell culture-based CHO residual DNA qPCR reaction.
The reagent savings of the six-fold volume reduction can be quite appealing on its own, but the increased level of sensitivity seen from the acoustically dispensed reactions using the Echo 655T and 555 Liquid Handlers provides a significant additional benefit to the resDNASEQ workflow. As seen in Figures 4D, 5D, and 6D, an accurate DNA quantitation was shown to be possible down to the 0.003 pg level for each kit tested.
The Echo 655T and 555 Liquid Handlers provide the ability to transfer small reagent volumes, which may be shifting the CP values on the standard curves to come out earlier as a higher concentration of DNA would be present in the reduced volumes. The increased level of sensitivity would be highly beneficial for reducing costly false positives in an industrial setting.
The final advantage presented by acoustic arraying was consistency across liquid handlers. The Echo 655T, 555, and 525 Liquid Handlers performed equivalently to the manufacturer proposed 0.03 pg LLOQ. The Echo 655T Liquid Handler has increased flexibility for scientists due to its ability to accept acoustic sample tubes, but still maintains the same proven precision seen in the Echo 555 Liquid Handler. The Echo 525 system had the added advantage of having a faster flow rate as compared to the Echo 655T and 555 systems, so it would be advantageous when a large number of samples are required. The mean percent CV for all ten experimental data sets was extremely low at 0.83%, providing increased confidence in the precision of each assay as compared to manual arraying. Also, the R2 value for each of the DNA standard curves was greater than 0.99 further confirming the transfer precision.
The importance of maintaining a contaminant-free pharmaceutical product is paramount. qPCR is an extremely sensitive assay that is well suited to determine potential host cell DNA contamination. Exponential amplification of DNA for signal is what drives the beneficial sensitivity of qPCR, but this same exponential characteristic can lend itself to greatly enhanced error when coupled with imprecise human-arrayed samples. In this study, the Echo 655T, 555, and 525 Liquid Handlers were demonstrated to produce extremely reliable data in a six-fold miniaturized reaction volume of three different resDNASEQ Quantitation Kits. Beyond reagent cost savings, the increased sensitivity afforded by acoustic dispensing can provide increased confidence in tested sample. Finally, direct dilution with the Echo Liquid Handlers decreased the potential for human introduced error when generating qPCR standard. In conclusion, the Echo liquid handling platforms provide the capacity for precise residual DNA testing using a reduced volume of the Applied Biosystems resDNASEQ Residual DNA Quantitation Kits.
Materials
| Equipment | Manufacturer |
| Echo 655T Liquid Handler | Beckman Coulter Life Sciences |
| Echo 555 Liquid Handler | Beckman Coulter Life Sciences |
| Echo 525 Liquid Handler | Beckman Coulter Life Sciences |
| Centrifuge 5430 | Eppendorf |
| Qubit | ThermoFisher |
| MixMate | Eppendorf |
| LightCycler 480 II | Roche |
| Reagents | Manufacturer | Part Number |
| resDNASEQ Human Residual DNA Quantitation Kit | Applied Biosystems | A26366 |
| resDNASEQ Quantitative CHO DNA Kit | Applied Biosystems | 4402085 |
| resDNASEQ Quantitative E. coli DNA Kit | Applied Biosystems | 4458435 |
| Penicillin Streptomycin | Gibco | 15140-122 |
| Fetal Bovine Serum | ATCC | SCRR-30-2020 |
| F12 (1x) + GlutaMAX Nutrient Mixture (Ham) | Gibco | 31765-035 |
| Qubit dsDNA HS Assay Kit | ThermoFisher | Q32851 |
| Consumables | Manufacturer | Part Number |
| Echo Qualified 384-Well Polypropylene Microplate 2.0 (384PP 2.0) | Beckman Coulter Life Sciences | 001-14555 |
| Hard-Shell 384-Well PCR Plates, Barcoded | Bio-Rad | HSR4805 |
| Microseal 'C' PCR Plate Sealing Film | Bio-Rad | MSC1001 |
| Qubit Microtube | ThermoFisher | Q32856 |
| 1.5mL DNA LoBind Tubes | Eppendorf | 22431021 |